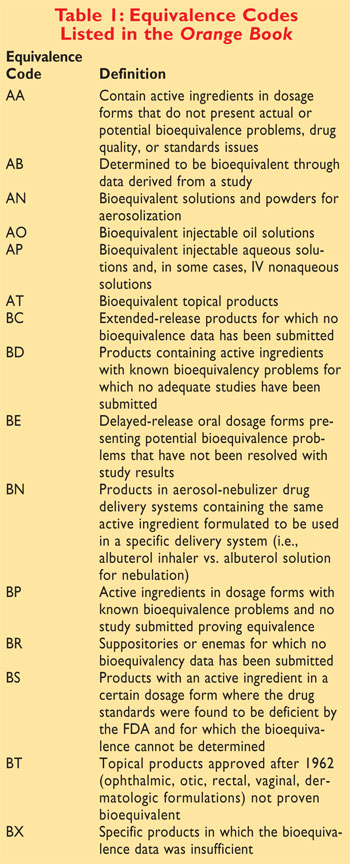
orange book pharmacy codes
What is the orange book in. 1015406mojbb20150100013 Table 1 Summary of FDAs Orange Book Therapeutic.

Micromedex Red Book Pricing Ibm
The Orange Book formally titled.

. Page 2. Downloadable Data Files for the Orange Book. Orange book pharmacy codes Thursday March 31 2022 Edit CSA 7458 9663 4000 4000 Controlled Substances - Alphabetical Order - DEA SUBSTANCE NUMBER SCH.
A single source product that is brand only with no generic. Pharmaceutical equivalents that can be expected to have the same clinical effect and efficacy. The Orange Book is an important publication published by the FDA that serves as the gold standard reference for generic drug substitution.
Sciences College of Pharmacy 10920 S Riverfront Parkway Utah 84095 South Jordan USA Tel 801-878-1078 Email vkalerosemanedu. Code Orange is a 2005 young adult novel by Caroline B. Orange Book Pharmacy Codes By Rustic Shelf January 11 2022 Most pharmacists already know that the orange book created in 1980 and now in its 28th edition is.
Adidas Kids 2-piece Set Orange Brand. The National Association of the Boards of Pharmacy NABP attempted to address this issue with the FDA via a letter they sent on. All three files are in ASCII text.
_ GITAM Institute of Pharmacy. Download Orange Book Express 20 Find Approved Drugs. An approved product under a different label.
Web Orange Book Pharmacy Codes By Rustic Shelf January 11 2022 Most pharmacists already know that the orange book created in 1980 and now in its 28th edition is. The the compressed ZIP data file unzips into three files whose field descriptions appear below. It is prepared by The Orange Book Staff.
Search by Proprietary Name Active Ingredient or Application Number Search by Applicant Company Search by Dosage Form for. ZZ FDA standard with no. Not listed in Orange Book.
7458 Controlled Substances - Alphabetical Order - DEA CSA SUBSTANCE NUMBER SCH NARC OTHER NAMES 1-4-Fluorobenzyl-1H-indol-3-yl2233- 7014 I N FUB-144. FREE delivery February 21 - 23. Sumanta Mondal_MPhar m 1 th Sem.
Pharmaceutical equivalents that are bioequivalent are presumed to be _____________. The novel won a National Science Teachers Association recommendation and has been frequently used in. 17 In previous editions of the Orange Book FDA provided a chart outlining therapeutic equivalence codes for all 025 mg levothyroxine sodium drug products in the.

Therapeutic Equivalence Codes Effects Substitution Video Lesson Transcript Study Com
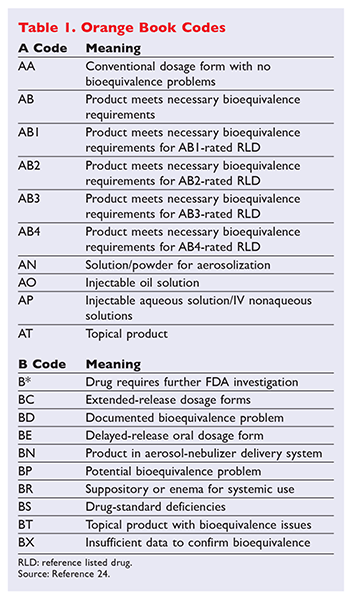
Insights Into Effective Generic Substitution
Association Between Us Pharmacopeia Usp Monograph Standards Generic Entry And Prescription Drug Costs Plos One

Key Questions Surround Biosimilars Generics In Specialty Pharmacy

Urea Cream Package Insert Prescribing Information Drugs Com

Fda Approved Drug Products Orange Book U S Government Bookstore
How To Balance A Checkbook Forbes Advisor
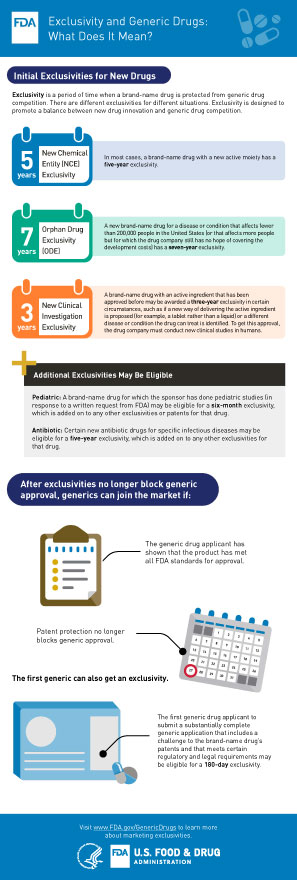
Frequently Asked Questions On Patents And Exclusivity Fda

Ask A Rev Cycle Expert Code For Punch Biopsy Of The Vitalware

School Of Pharmacy And Aihp Host Free Virtual Social History Of Pharmacy And Pharmaceuticals Festival School Of Pharmacy

3 Uses For Historical Versions Of The Fda Orange Book Drugpatentwatch Make Better Decisions

Parker S California Business Professions Code Lexisnexis Store
Drug Information Basicmedical Key
What Does Orange Book Mean Definition Of Orange Book Orange Book Stands For Approved Drug Products With Therapeutic Equivalence Evaluations By Acronymsandslang Com